
The aim of the GlioME team is to discover relevant targets, characterize their functional role in tumor growth and progression to develop innovative therapeutic strategies against glioblastoma (GBM).
GBM is the most frequent and aggressive primary brain tumor, with a median survival of 15 months after diagnosis (PMID: 34185076). GBM standard treatment consists in tumor surgery followed by radiotherapy and chemotherapy (PMID: 15758009). This protocol has been settled in clinic in 2005 and since the therapeutic strategy has not changed underlying an urgent need in new therapy development.
GBM resistance to treatment is mainly due to clinical and therapeutic challenges:
- the molecular and cellular heterogeneity of GBM including the presence of cancer stem-like cells (CSCs)
- the specificity of GBM tumor microenvironment surrounded by a blood-brain-barrier opposing drug penetration.
Our expertise combines imaging and phenotyping technics, modeling of preclinical models, development of tailored drug delivery systems and promotion of translational research to face these challenges. By having a bed-to-bench and a bench-to-bed approaches, we foster the transfer of our research to the clinic:
- a bed-to-bench approach: we identify relevant clinical targets from human samples (biological resource center, biobanking), investigate their role in tumor growth and progression, and decipher how they modulate the GBM microenvironment by developing relevant human-derived and mouse pre-clinical models.
- a bench-to-bed approach: we develop innovative therapeutic strategies from our human-derived and mouse pre-clinical models to early phase clinical trial.
These approaches are feasible thanks to:
- a fundamental/clinical leader-duet: Dr Aurélie Tchoghandjian, CRCN CNRS, expert in the study of gliomas, in particular GBM, specialized in developing technics to deeply study the tumoral microenvironment (clarification and light sheet imaging, multiparametric flow cytometry panels, two-photon imaging, spatial multiplexed proteomic) and with a long-lasting experience in the glioma pre-clinical models, mouse models and cultures of 3D models derived from GBM human samples (PDX and tumoroids). Prof. Emeline Tabouret, MD, PhD, Professor at Aix-Marseille University, medical oncologist specialized in neuro-oncology at the APHM, working in the Neuro-Oncology department and in the Phase I unit CLIP2 (Centre Labelisé INCa d’essais Phases Précoces), with a long-lasting experience in setting-up and coordinating pre-clinical and translational projects (HITCH NCT06045065, Gliomanoid NCT03971812, MMPredict NCT03526822, CIRANO) and academic phase I clinical trial for GBM patients (Proglio, PHRC-K 2019).
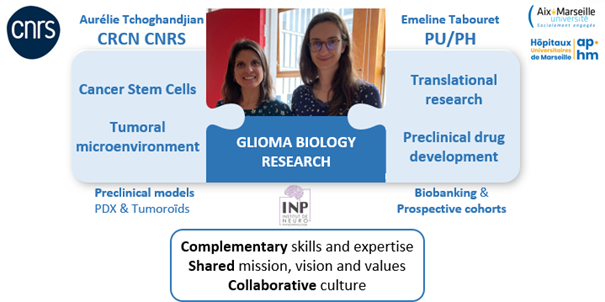
- the pluridisciplinarity of our team: the GlioME team gathers physician/lecturer experts in neuro-oncology (two neuro-oncologists, three pathologists, and one radiotherapist), three fundamental researchers (two CRCN CNRS and one MCU), and a large operational staff with two IR, five IE, one technician and one administrative officer. Moreover, the team currently host three doctoral students and one ATER.


Scientific background of the team
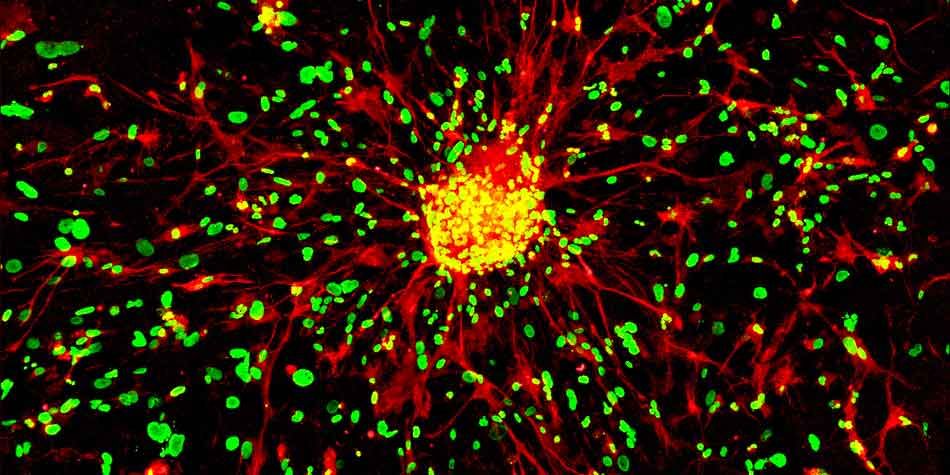
Our team previously showed that cells with cancer stem-like cells properties (CSCs) were identifiable in human glioblastoma (GBM) samples by using A2B5 and GD3 biomarkers (PMID: 19243384; 31466399; 35563061). Moreover, GBM also highly expressed ML-IAP, an inhibitor of apoptosis protein, which inhibition using SMAC mimetics was associated with a decrease of cancer cell stemness and prolonged survival of GBM mouse models (PMID: 30920104; 27490930). In addition, we showed that ML-IAP was expressed by the tumor-associated macrophages (TAMs), microglia cells and anti-inflammatory macrophages which increased after tumoral debulking (Preprint Snacel-Fazy et al.). Finally, our team recently showed that targeting these macrophages after surgery by using a combination of local treatment applied in the post-surgery cavity and SMAC mimetics increased mice overall survival (Preprint Bastiancich et al.).
Main objectives of the team
The main objectives of the team are now to unravel the implication of ML-IAP and GD3 in GBM PROGRESSION and MICROENVIRONMENT interactions to design specific inhibitors for a future THERAPEUTIC DEVELOPMENT as following:
1. FACTORS OF PROGRESSION: to investigate the functional role of key actors of GBM tumor growth and progression.
2. MICROENVIRONMENT: to determine relationships between GBM tumor core and its microenvironment during tumor growth and under treatment.
3. THERAPEUTIC DEVELOPMENT: to develop innovative therapies from our bench to the bedside of the patients.
To investigate the role of our targets, we compromise the expression of ML-IAP and GD3 by using chemical (SMAC mimetics [GDC-0152] for ML-IAP inhibition) and genetic approaches (shRNA/siRNA) in the further analysis.
- AIM 1: FACTORS OF PROGRESSION
Investigating the functional role of GD3 and ML-IAP in stemness
We perform clonogenic assays and clonal dilution to determine the self-renewal potential and the capacity of proliferation of GBM CSCs with compromise expression of ML-IAP or GD3. We also analyze their capacity of differentiation and how the modulation of the target expression impacts cell plasticity (divergent differentiation, expression of stemness, plasticity and differentiation markers, single cell RNA seq analyses). Finaly, we also investigate whether hypoxia could impair the role of our targets in stemness. Xenografts in nude mice are decisive to clearly validate the stemness potential of our targets.
- AIM 2: MICROENVIRONMENT
Determining relationship between GBM tumor core and its microenvironment by using in vitro / ex vivo 3D models
By using video-microscopy, we analyze the interactions between GBM tumor cells and cells of the microenvironment, tumor cell proliferation, tumor cell migration during 1) GBM growth, 2) at tumor resection and 3) in response to treatment (radiotherapy, chemotherapy, SMAC mimetics, drug combinations...). We use 3D models that we developed (models of co-cultures of astrocytes/microglia/ tumor cells; GBM explants; tumoroids) and organotypic slice models. By using immunostainings and flow cytometry experiments we determine the phenotype and the nature of the cells involved in the identified biological processes.
Determining relationship between GBM tumor core and its microenvironment through in vivo syngeneic mice models coupled to imaging systems and phenotyping approaches
We combine imaging and phenotypic approaches to determine how our targets could modulate the GBM microenvironment. We perform 3D light sheet imaging after whole mount staining and spatial proteomic to determine the densities, spatial distributions, and interactions of the cells of the microenvironment. To get a precise cellular composition of our samples we perform flow and mass cytometry. All these complementary technics allow a dissection and characterization of the immune cell subsets involved in 1) GBM growth, 2) in GBM recurrence and 3) in response to treatment.
Determining the impact of standard of care surgery on GBM microenvironment on tumor recurrences
We combine imaging and phenotypic approaches to determine how surgery can impact the onset and development of tumor recurrences. We perform tumor debulking in tumor-bearing mice and evaluate over time and space physiological modifications that might boost the proliferation of residual tumor cells. By characterizing the post-surgical microenvironment, we identify therapeutic windows and cellular targets that could be used to develop treatments to inhibit recurrences.
- AIM 3: THERAPEUTIC DEVELOPMENT (targeted therapies)
Designing and testing siRNA directed against the factors of progression that we have identified.
The final objective of our research is to design innovant therapeutic approach to target ML-IAP and GD3 pathway. We focus on siRNA approach and test their efficiency in decreasing tumor cell proliferation, invasion and stemness in in vitro, ex vivo and in vivo preclinical models.
Developing and evaluating optimized inhibitors of our selected target
We use up-to-date techniques to develop optimized inhibitors to antagonize ML-IAP to improve efficacy and selectivity of action. We test our drug candidates with our well settled 2D, 3D, ex-vivo and in vivo models.
Developing and evaluating drug delivery systems for GBM treatment
We overcome the therapeutic challenges of GBM treatment by developing tailored drug delivery systems for systemic or local administration. After identification of promising drugs, adapted drug delivery systems are designed based on the administration pathway and chemical nature of the molecules. The formulations are then developed, characterized physico-chemically and biologically and then tested using appropriate cellular and animal pre-clinical models.
Early phases clinical trial
We will transfer the most-promising therapy into early clinical phase thanks to our implication in the phase 1 department of Assistance-Publique des Hôpitaux de Marseille CLIP2 (Centre Labelisé INCa d’essais Phases Précoces).